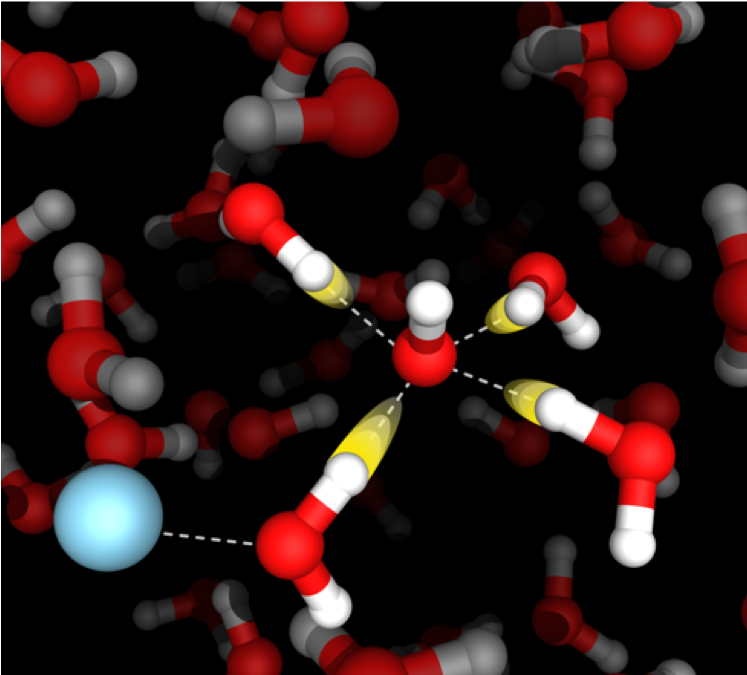
Aqueous acids, bases, and salts
Aqueous solutions containing acids, bases, and salts play an important role in many chemical, biological, and environmental processes. The Corcelli lab uses simulations to reveal the structure, dynamics, and transport of ions in aqueous electrolytes with atomistic resolution. In particular, this project aims to achieve a detailed molecular-level understanding of the role of counterions, ion-pairing, and solvent-shared ion pairs on the mobility of ions in solution, which is especially relevant to emerging battery technologies using aqueous electrolytes. State-of-the-art calculations of infrared spectra validate the simulations and uncover the spectroscopic signatures of structural and dynamical motifs in aqueous acids, bases, and salts.
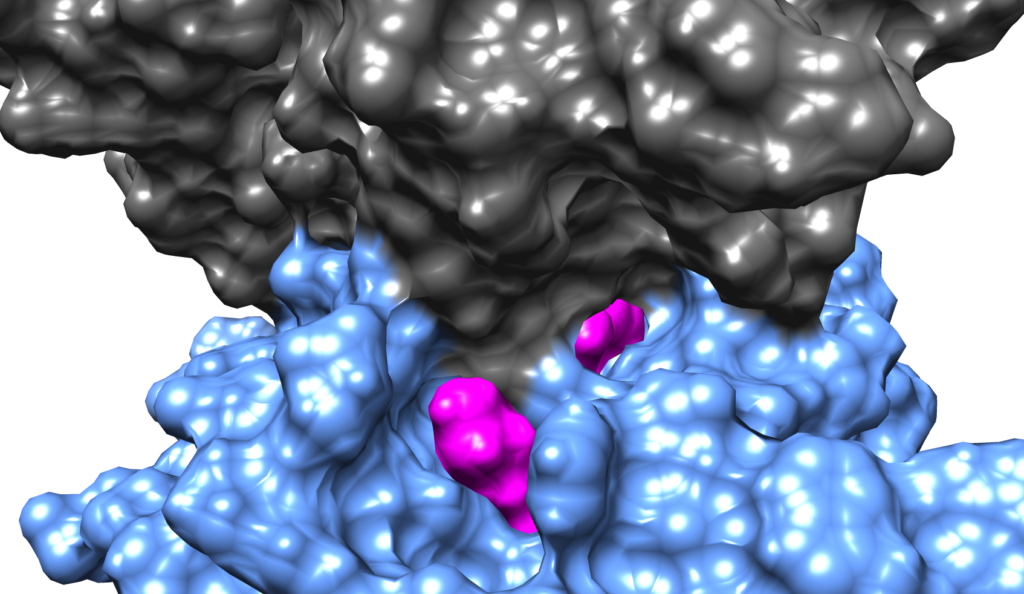
Biomolecular binding
The interactions of proteins with other proteins and the binding of proteins and RNA to DNA are essential to biological function. Moreover, the binding of small molecules to biomolecules forms the basis of many pharmacological interventions. However, the detailed molecular mechanism for these critical interactions and binding processes is not well understood. Leveraging graphics processor unit (GPU) acceleration and advanced sampling algorithms like the weighted ensemble method, the Corcelli lab is using atomistic simulations with explicit solvent to investigate the mechanism of drug complexation to DNA and the binding of a T cell receptor (TCR) protein to the major histocompatibility complex (MHC). These studies are elucidating the mechanism of biomolecular binding with unprecedented detail.