On the cell cortex, actin interacts with its upstream and downstream regulatory proteins in feedback loops. One interesting loop is the activator-inhibitor coupling between actin and the GTPase RhoA (or Rho), which is likely the most important protein in cytokinesis. As shown below, Rho produces actin through effector proteins, while actin inhibits Rho by recruiting RhoGAP.
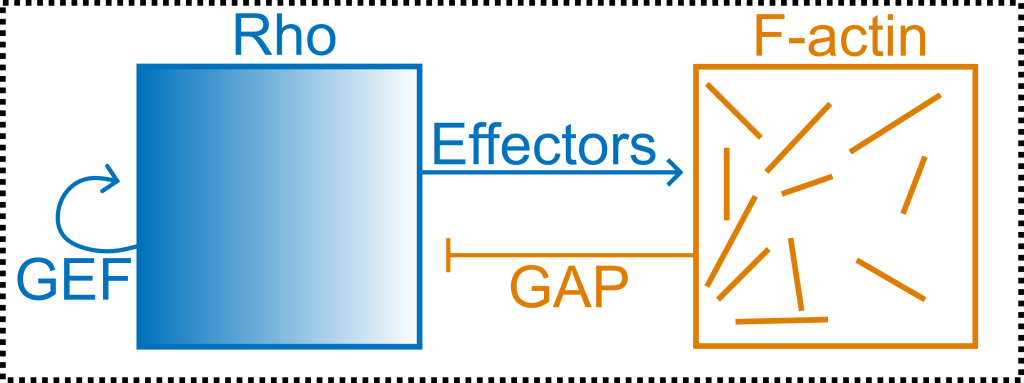
How does this coupling lead to self-organization of Rho and actin? It depends on the model system you look at. The two videos below show two different model organisms (starfish and C. elegans), with the same biochemical circuit shown above. In starfish, there are regular traveling waves of activity. In C. elegans, however, there are local transient pulses.
We are using models to try to understand how the different excitation patterns could result from actin kinetics. In initial work, we developed a hybrid model using discrete actin filaments and continuum Rho dynamics. This reproduced the basic behavior in frogs and C. elegans (see video below).
We are using these models to understand how actin assembly dynamics change from organism to organism, and how changes to actin assembly proteins (like formin and profilin) affect actin assembly and Rho activity. Our next goal is to use this framework to infer how other actin binding proteins affect actin assembly dynamics using cutting edge tools for data-based inference.