In polarity establishment and cytokinesis, cells use directional flow of actin and myosin to organize material and divide asymmetrically. The video below shows myosin (left) and F-actin (right) during the course of a single cell cycle in C. elegans.
We are interested in how these cell-scale “cortical flows” arise from smaller-scale interactions between actin networks, cross linkers, and myosin motors. It is known that cortical flows begin when myosin is locally inhibited on the cell cortex, but how these flows propagate in vivo is still not well understood.
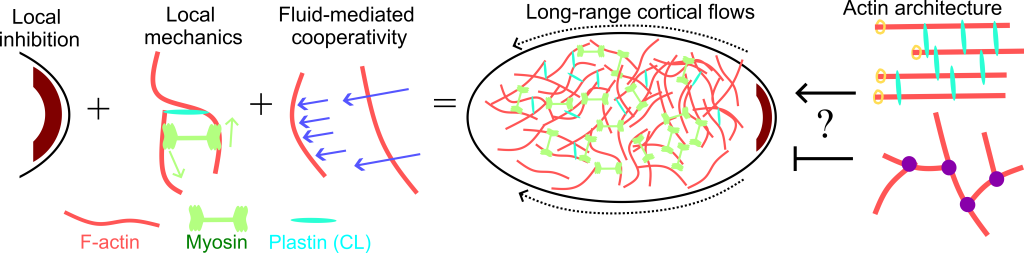
We are pursuing two main research questions in this area:
- Does flow of the background fluid help maintain or accelerate cortical flows? That is, should we think of cortical flows as a series of local force balances, or as many fibers being entrained in each other’s flow fields?
- What is the effect of different actin architectures on these flows? There is in vivo evidence that branched networks inhibit flow; can we understand the mechanism for this?
To do this, we are using in silico reproductions of cortical flows, as shown below.
Our future directions are to use our existing algorithms, which have the unique ability to simulate hydrodynamic interactions between fluctuating filaments, to understand how hydrodynamics contribute to positive feedback in cortical flows. We will also be extending our algorithms to account for branching in order to address the contribution of architecture.