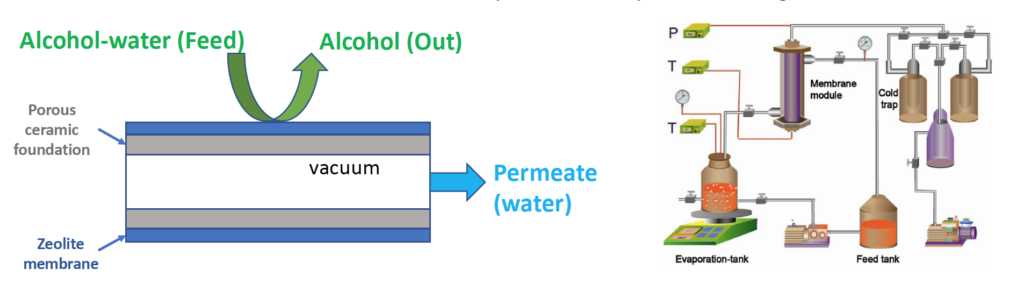
Membranes have been used to separate alcohol-water solutions (including azeotropic) and have proved to be energy and cost efficient over other methods. However, the membrane separation is often accompanied with poor performance, where molecular modeling is the perfect tool to elucidate insights and provide guidance on improvement.
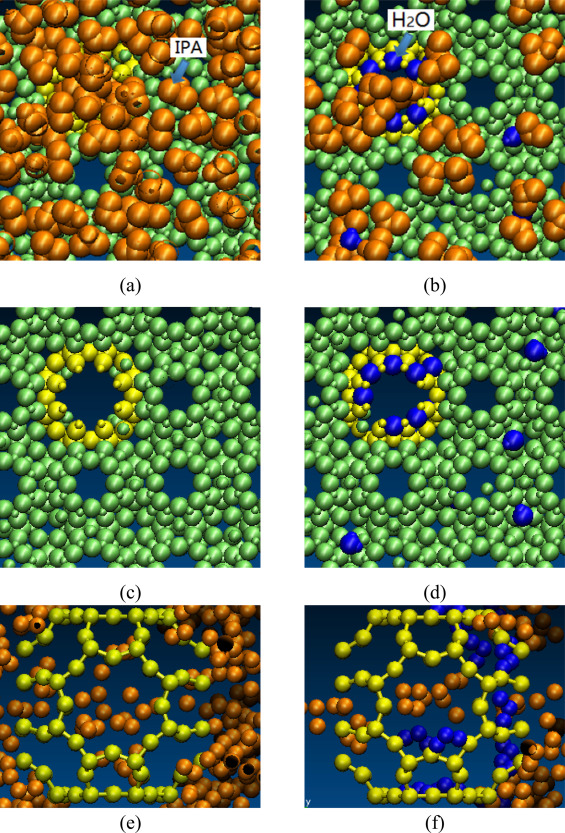
Why does the separation efficiency for isopropanol decrease at low water concentrations in the feed? Our simulation results revealed that the water molecules at the higher water concentrations gathered around and inside the defect pores on the zeolite membrane, which stopped the isopropanol from going through the membrane and had a positive effect on separation. At lower water concentrations there are not enough water molecules at equilibrium near or inside the defects to block these pores for possible IPA penetration.
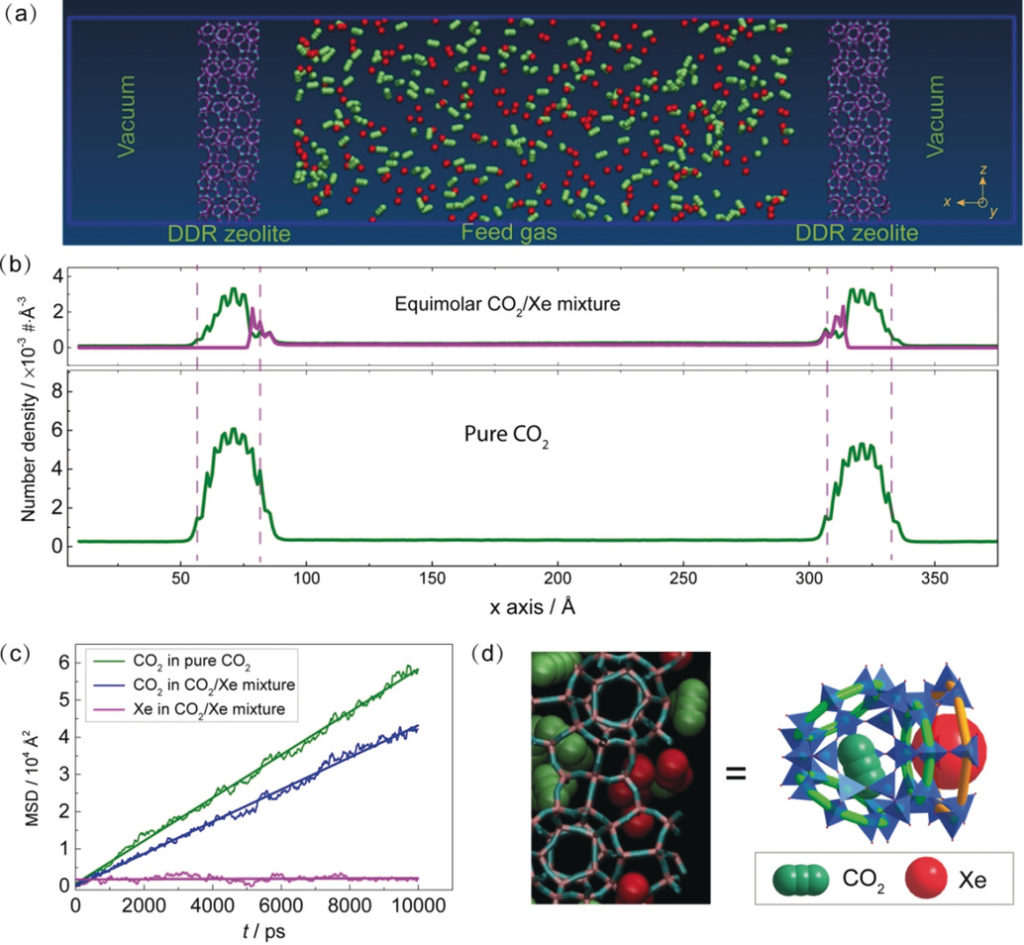
In the separation of CO2/Xe mixtures using DD3R zeolites, our simulations showed the adsorbed Xe atoms stayed on the surface cages and acted as a barrier for the adsorption and diffusion of CO2, leading to a lower diffusion rate of CO2.