Plasma catalysis is an improved conversion of the input gas flow by employing plasma and catalytic process. The applied electric field turns the gas into a conductor indicating the formation of plasma state with free electrons, excited molecules, and ions. The resulting low temperature, non-equilibrium plasma with energetic electrons interacts with the catalytic surface. Plasma-catalyst combination has a surplus effect, called synergy. Due to the synergistic effects of plasma catalysis, it has many applications including the destruction of volatile organic compounds, the production of fertilizers, the synthesis of value-added chemicals and the conversion of greenhouse gases. Many studies in the literature have presented an outstanding enhancement in the process of conversion. However, due to the multifaceted interaction between plasma and catalyst, the understanding of the fundamental mechanism is missing. In this study, we will focus on basic molecular interactions (e.g., adsorption, desorption) at the plasma-catalyst interface. The key outcome of this research will be the development of a novel reaction chamber in FTIR to investigate the interactions at the molecular level. The in situ FTIR studies will show how plasma species cooperate with catalyst and how those mechanisms are implemented to produce the desired products.
*written by Nazli Turan as a project summary
1. Introduction
Plasma enhanced chemistry is one of the most promising areas for environmental applications thanks to the characteristics of both plasma species and catalytic materials improving energy efficiency and product selectivity.1 Plasma catalysis has been used in a variety of applications from plasma enhanced chemical vapor deposition to the treatment of waste gas. 2,3 Compared to the conventional thermal processes requiring high temperature and high pressure, plasma processes are good candidates especially in the conversion of CO2, N2 fixation from the air and dry CH4 reforming.4 Most of the studies in the literature have shown experimental results implying an improvement in yield or conversion of gases with plasma catalysis, although the underlying mechanisms are not totally understood. The goal of this research is to provide a better understanding of plasma-catalyst interactions to produce the anticipated products. In the following sections, dielectric barrier discharges (DBDs) will be illustrated as a type of plasmas operated expediently in the applications of plasma catalysis. The role of catalysts will be clarified in a chemical reaction to elucidate the synergy between plasma and catalysis.
1.1. Definition and Characteristics of DBD Plasmas
Plasma is the fourth state of matter consisting of free electrons and ions. It is electrically conductive with the equal number of negative and positive charges. Plasmas are created by applying an electric or magnetic field to a gas to make it conductive. Gases can be fully ionized at high temperatures such as in fusion plasmas, although most laboratory plasmas are examples of partially ionized, non-equilibrium, low temperature plasmas with highly energetic electrons (), and less energetic ions and neutral gas particles ().5 The temperature of the electrons is much higher than the other particles because the applied electric field transmits energy to electrons instead of heating the gas as a whole. The energetic electrons collide with the gas particles resulting in excitation, ionization and dissociation. The collisions are probabilistic, and these probabilities are determined by the collision cross section, , which is a function of electron energy.
The collisions between electrons and neutrals are the essence of chemical reactions which are triggered by excitation and ionization.5 For example, the rate equation resulting in excitation can be written as , where Ne and Ng are the concentrations of electrons and gas particles, and kex is the reaction rate constant, , which is a function of the collision cross section and the electron velocity. It can be confidently asserted that the rate of excitation depends on the number density of electrons (concentration) and the temperature of electrons (energy). The number density and temperature of electrons define the type of plasma and which reactions can be driven. For instance, in microwave plasmas the dominant mechanism is vibrational excitation, while in dielectric barrier discharges the most profound mechanism is electron impact dissociation.6 The two abovementioned characteristics of electrons are obtained by solving Boltzmann transport equation (BTE) which is a seven-dimensional equation considering velocities and positions of the particles in time. An online Boltzmann solver, Bolsig+, is a helpful tool to determine the characteristics of electrons in a plasma.7 The input of the solver is the reduced electric field (E/Ng=E/p=V/pd) defining the energy gained between collisions, where E is electric field, p is pressure, V is the voltage difference and d is the characteristic gap.
The most used plasma type in industry is dielectric barrier discharges (DBDs) due to its easy setup and the operation at atmospheric pressure.8 DBDs are operated with alternating voltage in the frequency range of kHz to MHz. It is a self-sustaining plasma with an insulating material between the electrodes.9 The dielectric material is responsible for limiting current between alternating cycles.10
Although the applications of DBD plasmas have been attracting a great deal of attention in recent years, the very first experiment was conducted by Siemens in 1857.11 He investigated the generation of ozone in a coaxial design with alternating electric field.10 Plasma generated ozone has been largely used in drinking water plants aiming disinfection.12 With the experience gained during the production of ozone, plasma has been implemented to the treatment of gas flows and the destruction of volatile organic compounds (VOCs).13,14 Since DBDs have a straightforward design, they are also useful for large-scale industrial applications by running many DBD reactors in parallel.10,15
A typical DBD circuit consists of a high voltage power source with a frequency generator. Generally, a resistor and a capacitor are added to the circuit to measure the current and charge of the plasma. The plasma current (Fig.1a) is the sum of the displacement current (capacitive) and the filamentary current (resistive). Before breakdown, the plasma acts as a capacitor and only the displacement current is measured. Once the breakdown of the gas has occurred, the plasma acts as a variable resistor (Fig.1b).
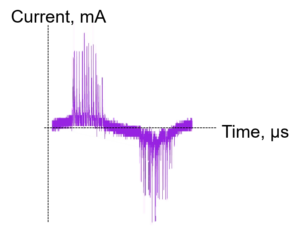
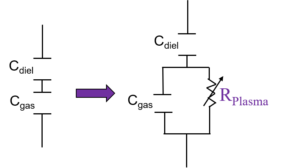
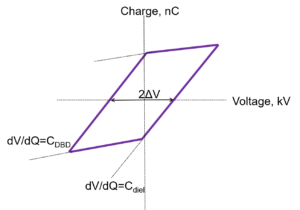
The total charge transmitted to the plasma can be measured with a voltage probe across the external capacitor which is in series with the plasma (). Charge vs. the applied voltage graph is represented by the Lissajous curve (Fig.2), the area of which represents the deposited energy to plasma per cycle. The power is calculated by multiplying the area of the curve by the applied frequency, .
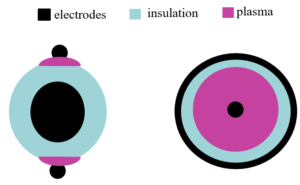
The DBD electrode geometries can vary with the desired operations. The planar or cylindrical configurations are frequently used in material processing and chemical conversion studies.16–18 The geometry can be adapted to form surface or volume DBDs. The basic schematics for surface and volume DBDs are shown in Fig. 3. Apart from geometry, these types of DBDs have different electrical characteristics which can be deduced from the number of filaments and the slopes of the Lissajous curve.9,19 Surface DBDs have more filaments and the Lissajous curve resembles a more elliptical shape implying the non-equal voltage thresholds with the varying gap between electrodes.20,21 Surface DBDs have shown superior performance for the removal of VOCs and the generation of ozone.19,22,23 As a novel geometry, we will introduce a helical DBD which can be characterized as a surface DBD. The main reason for using a helical geometry is to occupy less volume with electrodes which will be inserted into a reaction chamber in this project. A helical DBD setup consists of an insulated wire and an exposed wire which is wrapped around the insulated wire.
1.2. Catalysis
Catalysts are the materials that increase the rate of reaction by reducing the activation energy.24 The purpose of the implementations of plasma catalysis is to promote catalytic reactions by adding plasma in a reaction cycle.25 Transition metal catalysts (Ni, Fe, Co, Pt etc.) are employed to form bonds with the reactant molecules at the surface. Plasma catalysis is an example of heterogeneous catalysis which defines a solid catalytic surface interacting with gas phase molecules. Since a catalytic reaction occurs at the solid-gas interface, a large surface area is crucial to obtain an improvement in reaction rate.24 To increase the surface area, transition metal catalysts can be supported with porous metal oxides such as TiO2, Al2O3, or MgO. The support materials may influence the plasma behavior on the catalyst, since the dielectric constant of the material changes the distribution of charges along the dielectric surface.26
The metal catalysts on top of the supports affect adsorption/desorption of the gas molecules on the surface. The attachment of the gas phase reactants to the surface is called adsorption, and the detachment of the particles from the surface is called desorption. Adsorption, desorption and surface reactions occur at active sites where the reactants form bonds on the catalyst surface.24 The interaction between reactants and a metal catalyst follows the Sabatier principle which defines an optimum binding condition.25 The catalyst surface should stabilize the reactants to proceed a reaction. If the reaction is slow, the reactant/catalyst bond is either too weak or too strong.27 The effectiveness of the bonding between the reactants and the catalyst surface is deduced from the turnover frequency (TOF), which is the number of overall catalytic reactions per active site per unit time.25 As the first step, we are planning to probe the plasma-catalyst interactions with Ni catalyst on Al2O3 support. We will start with model systems (e.g., CO, CO2) where we can manage the gas compositions and the plasma environment to investigate adsorption/desorption mechanisms on plasma-activated surfaces. Further, we will study how to synthesize the desired hydrocarbons (CH4, C2H6) delicately by exploring the possible reaction pathways.
1.3. Mechanisms and Applications of Plasma Catalysis
Plasma catalysis refers to any combination of plasma and catalytic processes producing an enhanced conversion of the input gas flow.1 The gas is ionized by the applied electric field, and the resulting low temperature, non-equilibrium plasma interacts with the catalytic surface. The plasma species, including energetic electrons, vibrationally-electronically excited species, ions and radicals, take a role in surface reactions. Plasma catalysis differs from the thermal catalysis in operational conditions, specifically, under the given system pressure. While thermal catalysis requires extreme conditions such as vacuum or high pressure, plasma catalysis takes place in atmospheric conditions.25
Plasma catalysis exhibits an enhanced mechanism called synergy. Synergy defines a surplus effect which is greater than the sum of the plasma-alone and the catalysis-alone processes.25 The interaction can be observed in two ways. First, the plasma may have an impact on the catalyst by altering the characteristics of the catalyst such as the morphology and work function. Conversely, the catalyst may have an effect on the discharge characteristic of plasma by changing it partially discharged to fully discharged and by enhancing the electric field inside the pores.1,26,28 Additionally, both plasma and catalyst influence the surface processes by stimulating reactive species at the interface and by lowering the activation energy.25
Plasma catalysis has been applied to many areas. It has been widely used in the destruction of VOCs and in air treatment.29,30 CO2, a significant source of the greenhouse effect, has been studied to increase the rate of dissociation by synergistic effects.31,32 In addition to these, ammonia production has been investigated extensively due to its significance as a means of storage of hydrogen and as a source of nitrogen-based fertilizers.33–35 Our research group has shown that plasma catalysis yields comparable rates of ammonia synthesis to the high temperature, high pressure Haber-Bosch process.36
Dry methane reforming is another implementation of plasma catalysis and it has been studied intensively, since converting CO2 and CH4 to the composition of CO and H2 is a way to synthesize value-added chemicals such as hydrocarbons and methanol.8,37 The previous studies of our group on dry methane reforming showed that the plasma activity can be enhanced with catalysis at higher temperatures.38 The significant reduction in the energy barrier was observed in the case of plasma-catalyst coupling.39 The enhancement was attributed to the improved catalytic activity by changing surface chemistry, specifically, by affecting the rate of adsorption/desorption and by driving intermediate surface reactions. Although the conversion and selectivity studies endorsed the synergistic effect of plasma catalysis with macro-level thermodynamics and lumped kinetics, in situ measurements of the proceeding electrical/chemical/physical interactions between the plasma and catalysis are demanded to understand and control the coupling. Therefore, we will address molecular level interactions in this study.
2. Objectives and Research Strategy
Our aim is to obtain fundamental understanding of how plasmas modify surface chemistry and how these modifications can be exploited to control reaction kinetics, namely, adsorption, desorption, and intermediate surface reactions. We will reveal the actual chemical and physical details of the plasma-catalyst reactions by probing the molecular level to understand macroscopic behavior. For this purpose, we are developing a novel in situ and operando plasma Fourier transform infrared spectroscopy/mass spectrometry (FTIR/MS) platform. The characterization of plasma-surface interactions will be investigated with diffuse reflectance (DRIFTS) and attenuated total reflectance (ATR) configurations. Using electrical measurements, we will analyze the plasma characteristics and correlate these to FTIR measurements which will provide information about molecular bonds formed on the catalytic surface.
We are modifying the conventional reaction chambers of DRIFTS and ATR to account for the needs of a plasma environment. The modified design has additional wire electrodes to ignite a DBD plasma with a noble gas (He or Ar) while preserving the original optical design and preventing any possible damage to the FTIR components. With the modified reaction chamber, we will be able to run temperature programmed desorption (TPD) tests with and without plasma to obtain the energy barrier for desorption. Furthermore, we will study the interactions between the oxide supports and the probe molecules (CO, CO2) in FTIR, and then, we will investigate the surface interactions between the transition metal catalyst and the probe molecules.
3. Conclusion
Plasma catalysis is favorable for the production of value-added chemicals, waste treatment, the storage of energy and the conversion of greenhouse gases. Many studies have shown that there is a significant improvement in the yield of the resulting products or in the conversion of the harmful reactants compared to the conventional thermal processes. However, due to the complex interplay between plasma and catalyst, the underlying mechanisms are poorly understood. We aim to provide an essential molecular insight of plasma-catalyst interactions. The major outcome of this research will be the development of a novel tool to explore these interactions. Our in situ studies will indicate how plasma species couple with the catalyst. We will then examine the pathways of reactions in the presence of both plasma and catalyst to enhance the production of the desired products (e.g. CH4).
References
- Neyts, E. C. & Bogaerts, A. Understanding plasma catalysis through modelling and simulation—a review. J. Phys. Appl. Phys. 47, 224010 (2014).
- Whitehead, J. C. Plasma catalysis: A solution for environmental problems. Pure Appl. Chem. 82, 1329–1336 (2010).
- Kim, H.-H. Nonthermal Plasma Processing for Air-Pollution Control: A Historical Review, Current Issues, and Future Prospects. Plasma Process. Polym. 1, 91–110 (2004).
- Snoeckx, R. & Bogaerts, A. Plasma technology – a novel solution for CO 2 conversion? Chem. Soc. Rev. 46, 5805–5863 (2017).
- Go, D. B. Ionization and Ion Transport a primer for the study of non-equilibrium, low-temperature gas discharges and plasmas. (2018).
- Bogaerts, A., Kozák, T., van Laer, K. & Snoeckx, R. Plasma-based conversion of CO 2 : current status and future challenges. Faraday Discuss. 183, 217–232 (2015).
- Hagelaar, G. J. M. & Pitchford, L. C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 14, 722–733 (2005).
- Brune, L., Ozkan, A., Genty, E., Visart de Bocarmé, T. & Reniers, F. Dry reforming of methane via plasma-catalysis: influence of the catalyst nature supported on alumina in a packed-bed DBD configuration. J. Phys. Appl. Phys. 51, 234002 (2018).
- Brandenburg, R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 26, 053001 (2017).
10.Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 23, 1–46 (2003).
- Siemens, W. Ueber die elektrostatische Induction und die Verzögerung des Stroms in Flaschendrähten. Ann. Phys. 178, 66–122.
- Parvulescu, V. I., Magureanu, M. & Lukes, P. Plasma Chemistry and Catalysis in Gases and Liquids. (John Wiley & Sons, 2013).
- Clothiaux, E. J., Koropchak, J. A. & Moore, R. R. Decomposition of an organophosphorus material in a silent electrical discharge. Plasma Chem. Plasma Process. 4, 15–20 (1984).
- Fraser, M. E. & Sheinson, R. S. Electric discharge-induced oxidation of hydrogen cyanide. Plasma Chem. Plasma Process. 6, 27–38 (1986).
- Bogaerts, A. & Neyts, E. C. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Lett. 3, 1013–1027 (2018).
- Eden, J. G. et al. Plasma Science and Technology in the Limit of the Small: Microcavity Plasmas and Emerging Applications. IEEE Trans. Plasma Sci. 41, 661–675 (2013).
- Becker, K. H., Schoenbach, K. H. & Eden, J. G. Microplasmas and applications. J. Phys. Appl. Phys. 39, R55–R70 (2006).
- Ozkan, A. et al. The influence of power and frequency on the filamentary behavior of a flowing DBD—application to the splitting of CO 2. Plasma Sources Sci. Technol. 25, 025013 (2016).
- Nassour, K. et al. New Hybrid Surface–Volume Dielectric Barrier Discharge Reactor for Ozone Generation. IEEE Trans. Ind. Appl. 53, 2477–2484 (2017).
- Enloe, C. L. et al. Mechanisms and Responses of a Single Dielectric Barrier Plasma Actuator: Plasma Morphology. AIAA J. 42, 589–594 (2004).
- Gibalov, V. I. & Pietsch, G. J. The development of dielectric barrier discharges in gas gaps and on surfaces. J. Phys. Appl. Phys. 33, 2618–2636 (2000).
- Nobrega, P. A. et al. Comparison between performances of surface and volume nanosecond pulsed dielectric barrier discharges for the treatment of volatile organic compounds. 23rd International Symposium on Plasma Chemistry – ISPC 23, hal-01524685 (2017).
- Nassour, K. et al. Comparative Experimental Study between Surface and Volume DBD Ozone Generator. Ozone Sci. Eng. 38, 70–76 (2016).
- Fogler, H. S. Essentials of Chemical Reaction Engineering (Pearson Education, 2010).
- Neyts, E. C., Ostrikov, K. (Ken), Sunkara, M. K. & Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 115, 13408–13446 (2015).
- Zhang, Q.-Z., Wang, W.-Z. & Bogaerts, A. Importance of surface charging during plasma streamer propagation in catalyst pores. Plasma Sources Sci. Technol. 27, 065009 (2018).
- Masel, R. I. & Masel, R. I. Principles of Adsorption and Reaction on Solid Surfaces. (John Wiley & Sons, 1996).
- Butterworth, T., Elder, R. & Allen, R. Effects of particle size on CO 2 reduction and discharge characteristics in a packed bed plasma reactor. Chem. Eng. J. 293, 55–67 (2016).
- Jia, Z. & Rousseau, A. Sorbent track: Quantitative monitoring of adsorbed VOCs under in-situ plasma exposure. Sci. Rep. 6, (2016).
- Jia, Z., Wang, X., Thevenet, F. & Rousseau, A. Dynamic probing of plasma-catalytic surface processes: Oxidation of toluene on CeO 2. Plasma Process. Polym. 14, 1600114 (2017).
- Mei, D., Zhu, X., He, Y.-L., Yan, J. D. & Tu, X. Plasma-assisted conversion of CO 2 in a dielectric barrier discharge reactor: understanding the effect of packing materials. Plasma Sources Sci. Technol. 24, 015011 (2014).
- Zhang, K., Zhang, G., Liu, X., Phan, A. N. & Luo, K. A Study on CO 2 Decomposition to CO and O 2 by the Combination of Catalysis and Dielectric-Barrier Discharges at Low Temperatures and Ambient Pressure. Ind. Eng. Chem. Res. 56, 3204–3216 (2017).
- Gómez-Ramírez, A., Cotrino, J., Lambert, R. M. & González-Elipe, A. R. Efficient synthesis of ammonia from N 2 and H 2 alone in a ferroelectric packed-bed DBD reactor. Plasma Sources Sci. Technol. 24, 065011 (2015).
- Peng, P. et al. Atmospheric Pressure Ammonia Synthesis Using Non-thermal Plasma Assisted Catalysis. Plasma Chem. Plasma Process. 36, 1201–1210 (2016).
- Hong, J. et al. Plasma Catalytic Synthesis of Ammonia Using Functionalized-Carbon Coatings in an Atmospheric-Pressure Non-equilibrium Discharge. Plasma Chem. Plasma Process. 36, 917–940 (2016).
- Mehta, P. et al. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 1, 269–275 (2018).
- Snoeckx, R., Zeng, Y. X., Tu, X. & Bogaerts, A. Plasma-based dry reforming: improving the conversion and energy efficiency in a dielectric barrier discharge. RSC Adv. 5, 29799–29808 (2015).
- Kim, J., Go, D. B. & Hicks, J. C. Synergistic effects of plasma–catalyst interactions for CH 4 activation. Phys. Chem. Chem. Phys. 19, 13010–13021 (2017).
- Kim, J., Abbott, M. S., Go, D. B. & Hicks, J. C. Enhancing C–H Bond Activation of Methane via Temperature-Controlled, Catalyst–Plasma Interactions. ACS Energy Lett. 1, 94–99 (2016).