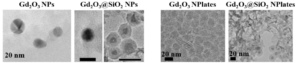
Well dispersed Gadolinium oxide nanoparticles and nanoplates were synthesized. A silica shell was used to increase biocompatibility.
Nanoparticles (NPs) due to their size scale being 1-100 nm, are characterized by a greater surface area per unit mass than the bulk phase of the same material, which makes NPs display chemical reactivity and physical properties that approach the atomic phase.
NPs derived from lanthanides (e.g. gadolinium, europium, etc.) in particular attract a lot of attention because of their unusual optical, magnetic, electrical, and catalytic properties. For instance, gadolinium oxideNPs are used as positive contrast agent in T1 weighted MRI scans due to the unique magnetic properties of gadolinium.
At present, commonly employed approaches for the synthesis of gadolinia NPs involve thermal decomposition techniques or metal co-precipitation protocols. While these methods yield nanocrystal units in the order of 5-20 nm, the actual hydrodynamic radius is a 100 fold higher (500-1000 nm). The high hydrodynamic radius causes colloidal agglomeration in solution. This makes these NPs highly unsuitable for in vivo biomedical applications or incorporation into 3D printing inks.
Our ongoing research has tackled this problem of super-agglomeration of metal oxides in solution by synthesizing non-sintered, well separated, water dispersed nanocrystals of metal oxides (iron oxde, gadolinium oxide and hafnium oxides). Our synthesis technique has broad applications in creating metal pigment inks for additive manufacturing. Additionally, we possess the ability to incorporate these metal oxide cores in biocompatible shells such as silica to make them suitable for in vivo applications.
Our paper detailing this synthetic technique will be published this Fall, 2017


Recent Comments