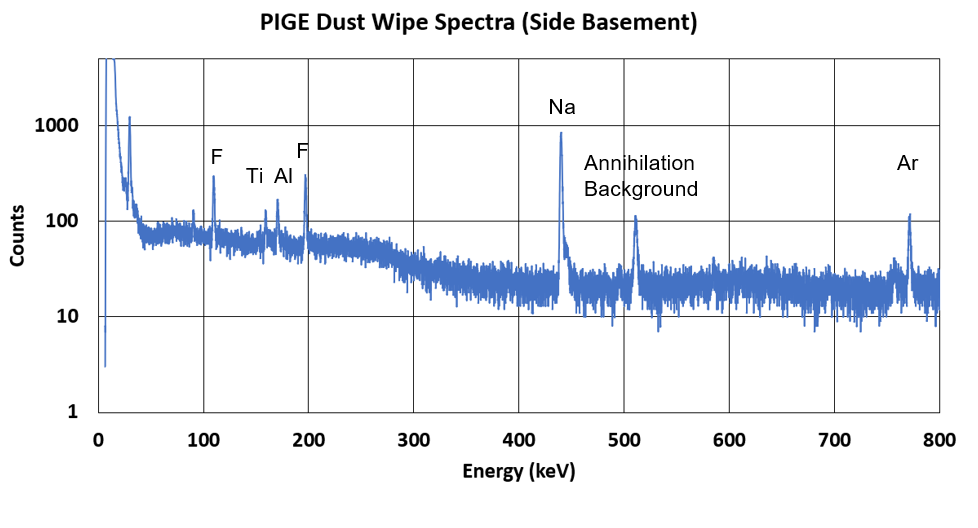
Proton-Induced Gamma Emission (PIGE) is a nuclear excitation technique commonly used to identify elements with atomic numbers (Z) less than 20. One element of particular interest is fluorine, which is characterized by 109 and 197 keV gamma rays. While these peaks provide an indication of the total fluorine content in a sample, fluorine has gained significance due to the study of Per- and Poly-fluoroalkyl Substances (PFAS), a widespread environmental contaminant with negative health impacts on humans. Other elements that can be identified using PIGE include silicon (Si), aluminum (Al), phosphorus (P), sodium (Na), magnesium (Mg), and chlorine (Cl).
Below is a representation of what happens in this process. Note that blue circles are protons and gray circles are neutrons. The initial blue circle (the one of the far left) is coming in with energy from a particle accelerator!
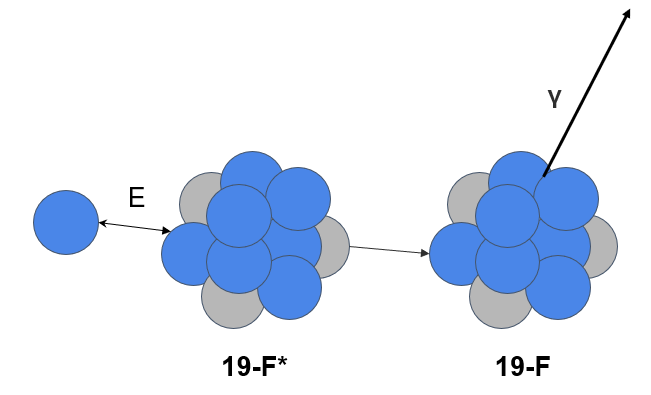
Here is an example of what’s called a spectrum from these measured gamma rays being emitted from one of our samples. This is a small snippet of the spectrum showing a few of the elements we commonly look for with PIGE.