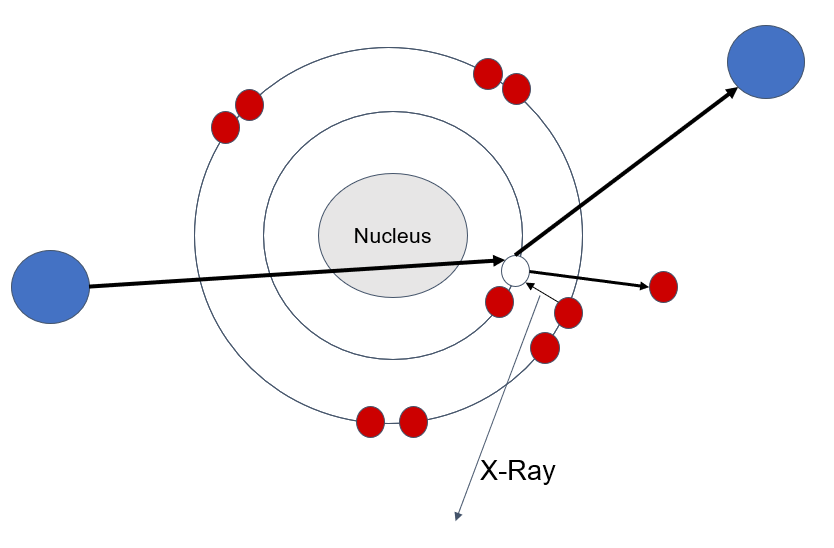
Proton-Induced X-ray Emission (PIXE) is a similar technique to PIGE that operates on an atomic scale. In PIXE, protons excite inner electrons, creating vacancies that are subsequently filled by outer-shell electrons, resulting in the emission of characteristic x-rays specific to the emitting atom. Each element has multiple characteristic x-ray energies, determined by the involved outer and inner shells. The observed x-rays represent transitions to the n=1 or n=2 states, known as K or L transitions. Transitions from a state one level higher are called alpha transitions, while transitions from two levels higher are referred to as beta transitions. These characteristic x-rays occur at significantly lower energy levels compared to the gamma rays measured in PIGE. To enhance the detectability of these x-rays, their energies must typically fall within the 4-26 keV range, which is predominantly found in elements with atomic numbers larger than 20, except for a few cases where the K line is too intense and the L line is too weak. Elements that can be identified using PIXE analysis include titanium (Ti) to zirconium (Zr) based on their atomic numbers, as well as certain high atomic number elements such as lead (Pb). By employing both PIGE and PIXE analysis simultaneously, we can effectively probe most elements of interest as they cover different regions of the periodic table.
Below is a representation of what this process looks like. The blue circles are protons, red are electrons. The initial blue circle (on the far left) is coming in with some energy from a particle accelerator for example.
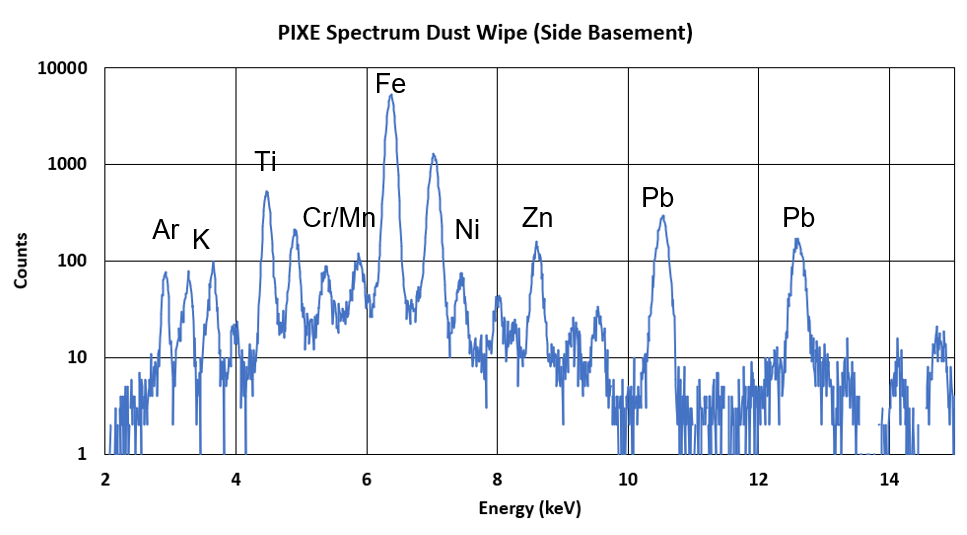
Below is a spectrum from our PIXE detector, showing most of the range that we see with elements labeled in their peaks.