Despite unstinting interventions such as chemotherapy and surgical tumor removal, approximately 40% of patients with stage I-III triple-negative breast cancer (TNBC) will experience tumor recurrence. Fortunately, not all hope is lost. The advent of immune checkpoint blockade (ICB) immunotherapy—a type of therapy that uses one’s own immune cells to kill the cancer—has shown great promise for the treatment of TNBC. However, as Dr. Azra Raza says in her book, The First Cell: And the Human Costs of Pursuing Cancer to the Last, these “immune approaches are not universally curative and, at present, help very few patients.”
Indeed, a significant number of TNBC patients do not respond to ICBs, and data from clinical trials evaluating the use of ICBs to treat TNBC have been conflicting. Because of this, researchers have been keen on understanding why some TNBC patients respond poorly to ICB therapy. The answer may lie in tumor biomechanics, and it might serve as one avenue for lowering the rate of tumor recurrence in TNBC patients.
Understanding tumor biomechanics
While cancer has been traditionally looked at through a biological lens, the pathological role of tumor mechanical forces has gained increasing attention. What do I mean by that? Imagine yourself in a room made up of rubber walls that is as tall and wide as you. Now, imagine that you begin to grow so much that you start pushing against and elastically deforming the walls around you. Naturally, as you push against these walls, they will push back against you. Over the past few years, these mechanical interactions are exactly what cancer researchers have begun to consider in tumor growth; instead of you in the room, it’s a tumor, and the walls surrounding the tumor are extracellular matrix components (ECM) (e.g., collagen, hyaluronan), blood vessels, fibroblasts, and normal adjacent tissue (NAT). The phenomenon I just described, albeit simplistically, is called solid stress, and it has profound implications in cancer progression, especially in relation to the immune response.
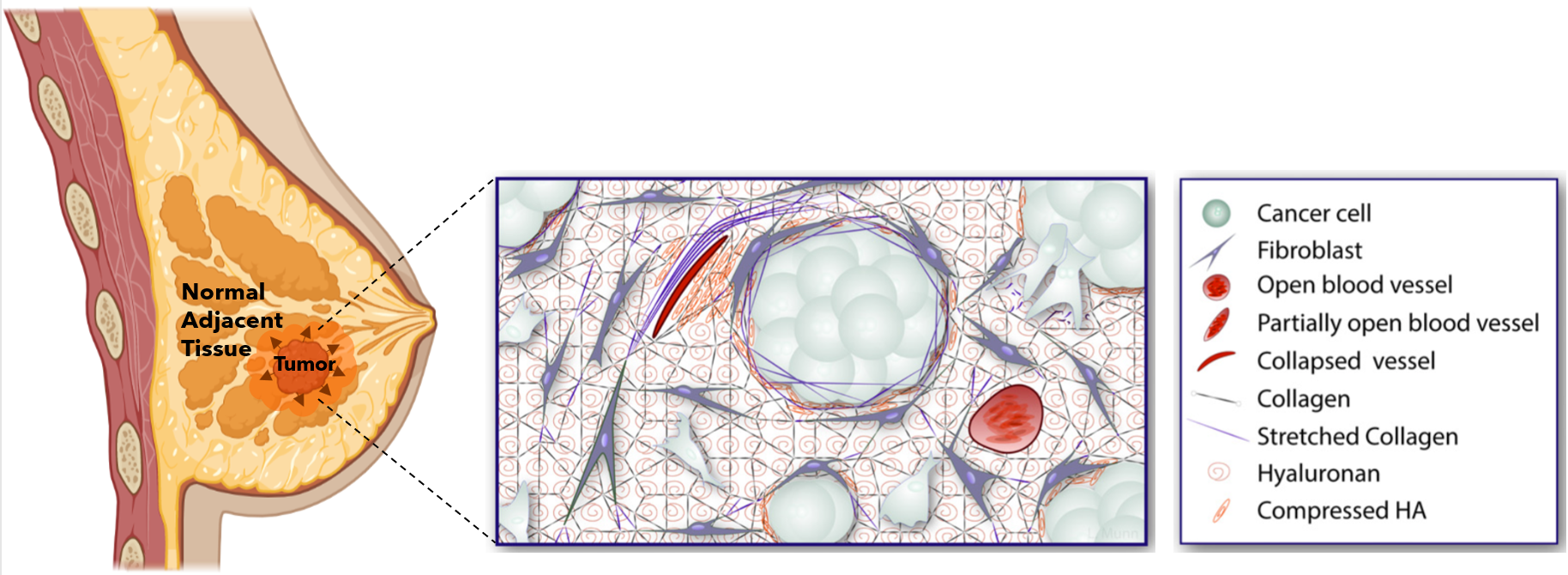
How does solid stress affect response to ICB immunotherapy?
Solid stress has been shown to create an immunosuppressive environment around the tumor. What that means is that the immune cells that are supposed to fight off the cancer are unable to do so. More importantly, this also means that immune therapies like ICBs are rendered useless without these fighting soldiers. Recently, however, researchers have found that targeting the origins of intratumoral solid stress (i.e., the effects of solid stress on the tumor) can actually improve the response to immunotherapeutic treatment in mice with TNBC (shown below).
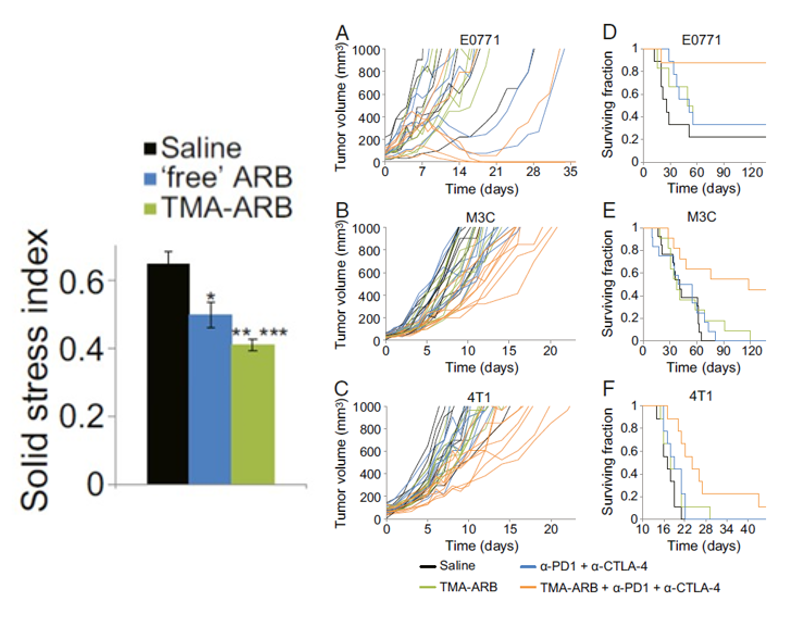
Can targeting extratumoral solid stress lower TNBC recurrence after surgery?
While researchers have investigated intratumoral solid stress, the effect of solid stress on the NAT (extratumoral solid stress) has yet to be uncovered for TNBC, especially after tumor removal. Current evidence has shown that the fraction of cancer associated fibroblasts (CAFs) tends to increase the further you stray from the tumor into the NAT. This is important because CAFs are immunosuppressive and have been shown to be activated by solid stress. Therefore, it is possible that the lingering effects of solid stress on the NAT are conferring immunotherapeutic resistance, even when a tumor is no longer present. As we look towards the future, it is possible that understanding these chronic effects may enhance patient response to ICB immunotherapy after surgical tumor removal and, consequently, help lower the rate of TNBC recurrence.
