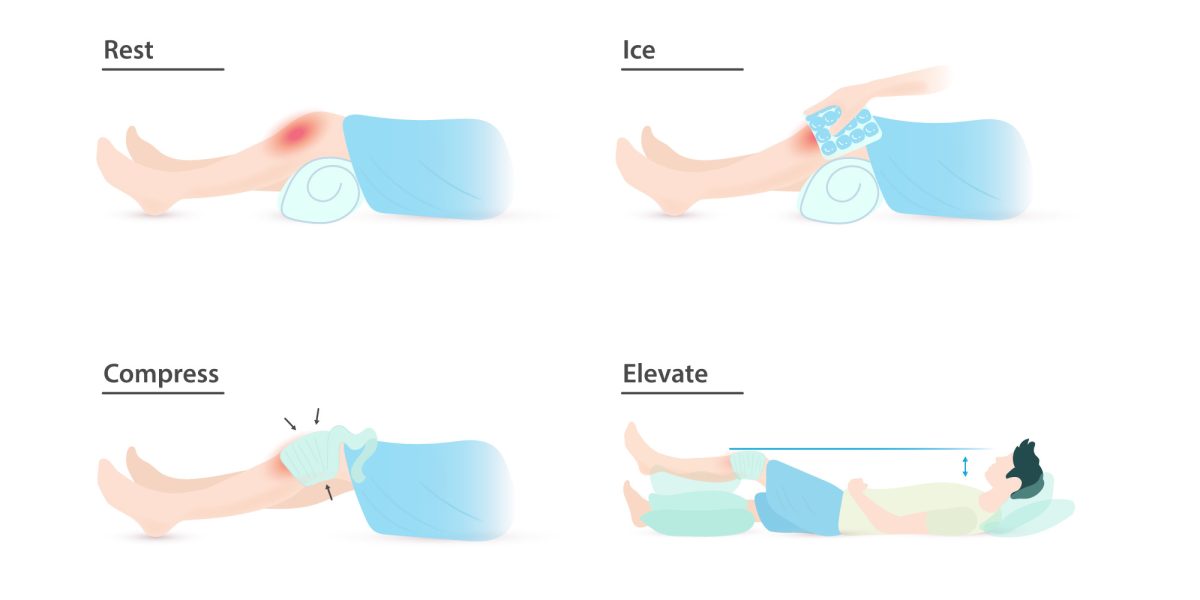
Have you ever been instructed to use the RICE protocol? Maybe you twisted your ankle on a root, slipped and fell hard on a patch of ice, or pulled your hamstring in an intramural soccer match. Rest, Ice, Compression, and Elevation is the common advice for immediate management of a soft tissue injury. But when you wrap a swollen calf, cover it with ice, and prop it on a pillow, what is actually going on beneath the skin? You may be able to feel the numbing cold of the ice and the compressive pressure of the wrap, but what about the healing processes that are harder to distinguish?
Continue reading “What are the RICE Method’s Impacts on the Healing Process Following Muscle Injuries?”Tag: healing
The Ultimate 2-for-1: the Power of Contralateral Strength Training
For the competitive athlete, injury often means loss. Loss of playing time, loss of skill development, and most importantly, loss of training time. These are all unfortunate consequence of getting a bone or tissue injury requiring a long-term healing prognosis. Injuries can be so devastating because the road to recovery is often times an arduous two-step process. First, the athlete must wait for their broken bones, torn ligaments, or pulled muscles to naturally heal. During this time, the athlete’s injured limb is likely immobilized in a cast or brace, leaving the resulting muscle to slowly atrophy as the body tries to heal itself. As a result, an athlete must spend the second part of their recovery process re-training the weakened muscles in the immobilized limb to return to full-strength. What if there was a way to heal and train the body at the same time? This is the power of a neurophysiological phenomenon known as “contralateral strength training.”
Continue reading “The Ultimate 2-for-1: the Power of Contralateral Strength Training”How Mice Could Help You Regenerate a Lost Limb
If you have ever experienced a nasty scrape or burn, you know the process of healing is not very fun. Human skin can take several weeks to regenerate after an injury and that often comes with a fair amount of pain. For a bigger injury that involves tissue damage, there is often little the human body can do to regenerate larger parts. However, thanks to a small rodent – the African spiny mouse – regenerative medicine for humans could be making huge advances in the near future.
Continue reading “How Mice Could Help You Regenerate a Lost Limb”Why your scar tissue isn’t an issue
What do knee scrapes, adolescent acne, and paper cuts have in common? They all have the potential to leave a nasty scar. For people who have undergone trauma that results in serious wounds, especially on the face, scar aging is a serious concern. What are scars, and why does scar tissue tend to look different than regular skin as aging occurs?
Continue reading “Why your scar tissue isn’t an issue”Ankle Sprains: An Epidemic in the World of Athletics
Have you ever been out running on a gorgeous fall day, only to have the run cut short by a painful misstep on a tree root covered by leaves? I have, and let me tell you – it’s awful! And even if you aren’t a runner, according to the Sports Medicine Research Manual, ankle sprains are a common, if not the most common, injury for sports involving lower body movements. Now, the solution to preventing this painful and annoying injury could be as simple as avoiding tree roots and uneven ground, but the real problem behind ankle sprains deals with the anatomy of the ankle.
Continue reading “Ankle Sprains: An Epidemic in the World of Athletics”Why do bone fractures take a long time for healing?
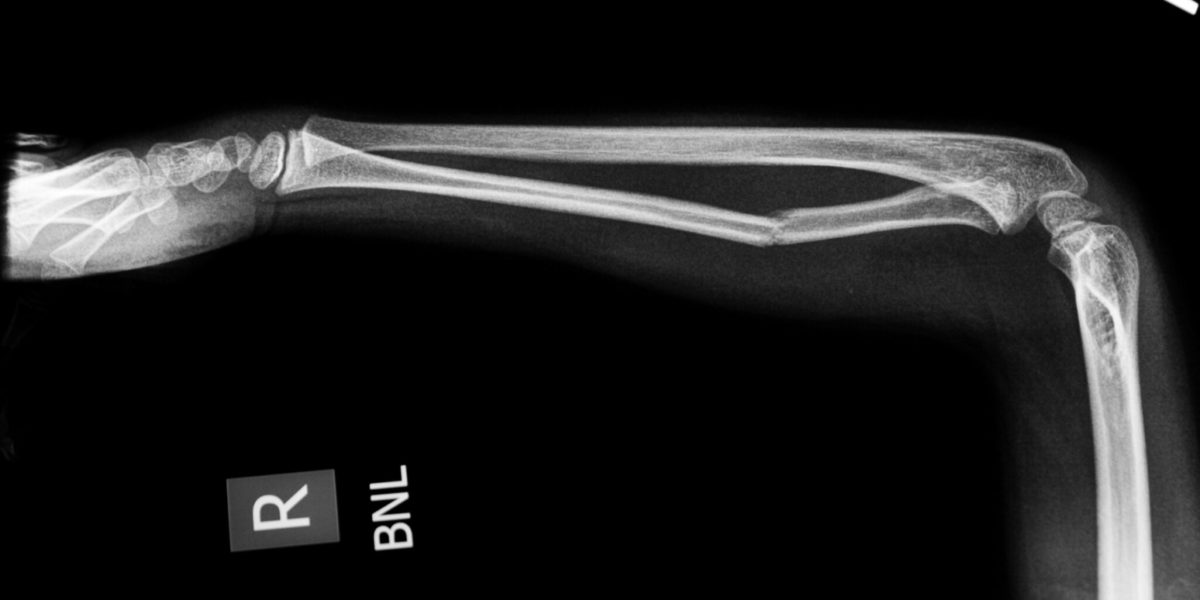
Have you observed that someone around you has broken their arms or legs? Bone fracture is a complete or incomplete break of bone continuity. And it is very common in our daily lives that there are more than 3 million cases in the U.S. per year. Many events may cause bone fractures, such as falls, car accidents or sports injuries. So, do you know how long it takes for the fracture to heal?
Continue reading “Why do bone fractures take a long time for healing?”Skeletal Support Seekers’ Success (So Far)
Bones break, and broken bones need time to heal, or regrow. Fans of J.K. Rowling’s Harry Potter series are quite familiar with the concept of bone repair, as Harry is once required to drink a Skele-Gro potion to magically (and painfully) regrow his arm bones overnight. Now, as fantastic as it would be to completely fix broken bones in a few hours, modern medicine has not yet discovered that secret of the Wizarding World; however, several treatments have been developed in attempts to speed the rate of fracture repair as well as increase the comfort of the patient (take that, Skele-Gro).
Continue reading “Skeletal Support Seekers’ Success (So Far)”