When we think of building strong bones, most of us picture drinking plenty of milk for its calcium—at least, that’s what our elementary gym teachers used to tell us. But building and maintaining strong bones requires more than just calcium from milk; supplemental vitamins play a key role in the development and maintenance of strong bones. Bone fractures are common in young, but especially elderly people, and adding collagen supplements to one’s diet can have a major impact on bone toughness reducing the risk of fractures.
Continue reading “Taking Collagen and Copper Supplements can decrease the risk of bone fractures”Tag: bone
Diving Deep into the Depths: Exploring Biomechanical Adaptations of Deep-Sea Creatures
If you’ve ever been at the bottom of a deep pool or body of water, then you’ve been able to feel some effects of water, also known as hydrostatic, pressure. Your ears begin to pop, your nasal cavity starts to feel a lot of pressure, and your eyes begin to feel compressed. Now imagine diving 10,000 ft deep, where you’d feel 300 times the pressure you would feel during that small dive. Your bones would begin to crush and crack, your lungs would collapse, and much more. We still know very little about the ocean–it is said that we know more about outer space than the ocean–but as we keep exploring, we learn more about different deep sea creatures–aquatic animals residing over 1,000 m below sea level–and how they survive such immense hydrostatic pressure at abysmal depths. By discovering more about their physical adaptations, we can design better vehicles or modes of withstanding these high pressures to venture deeper into the sea. So, how do these creatures survive such immense pressures? What do they have biomechanically that we don’t possess?
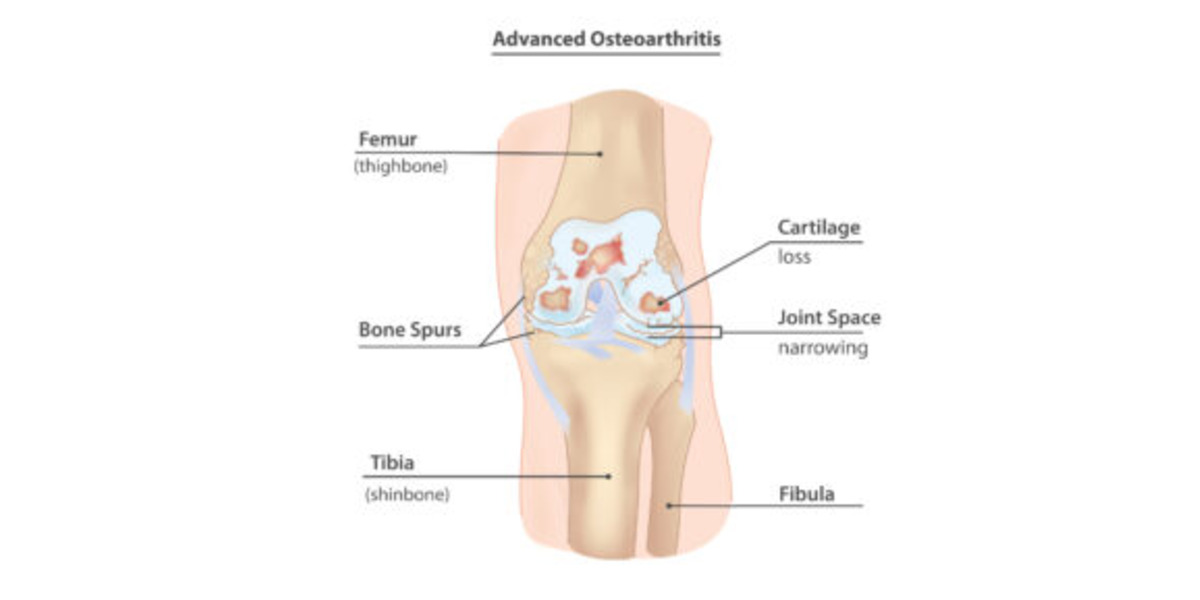
From Strain to Pain: Role of Altered Loadings at Joints as Onset for Osteoarthritis (OA)
Featured image courtesy of InjuryMap.
Joint degenerative diseases, such as Osteoarthritis, are an increasing cause of pain and disability in millions of people worldwide, per Sanchez-Adams et. al. As a culture of intense, nonstop work is promoted, people incline to not give their bodies enough time to rest, which has increased the cases of joint injuries, especially those to the lower body; specifically in those populations that are exposed to high intensity physical activity. This is concerning, since it has been discovered that joint injuries provoke an alteration in how these joints distribute loads, drifting from their physiological, or typical/natural, behavior, as demonstrated by Ko et. al. Consequently, this affects the biological response of the bone and cartilage that compose that affected joint, leading to potential degeneration due to the altered mechanotransduction (interpretation of mechanical signals to biochemical ones) interactions of the cell and cartilage forming cells, osteocytes and chondrocytes. This degeneration is what characterizes the concerning and debilitating Osteoarthritis (OA). This leads us to our questions: Why does the altered loading affect my joints? If I suffer an injury, can I prevent the degeneration of my joints and live a long, healthy life?
Continue ReadingOsseointegrated Prosthetic Devices: What Are They and How Did We Get Here?
Prosthetic devices have been an option for limb-different individuals for thousands of years. Over the centuries, there have been countless discoveries that have led to the modern prosthetic leg. Today’s customary prosthetic leg is comprised of two main components: the socket and the device itself. The socket is often made of carbon fiber and is molded to the contours of the user’s residual limb. This socket is directly connected to the device itself, which includes an ankle and possible knee joint depending on the length of the limb. The socket serves as the connection point between the user’s residual limb and the prosthetic leg.
Continue reading “Osseointegrated Prosthetic Devices: What Are They and How Did We Get Here?”Nature vs. Nurture: who is responsible for bone shape?
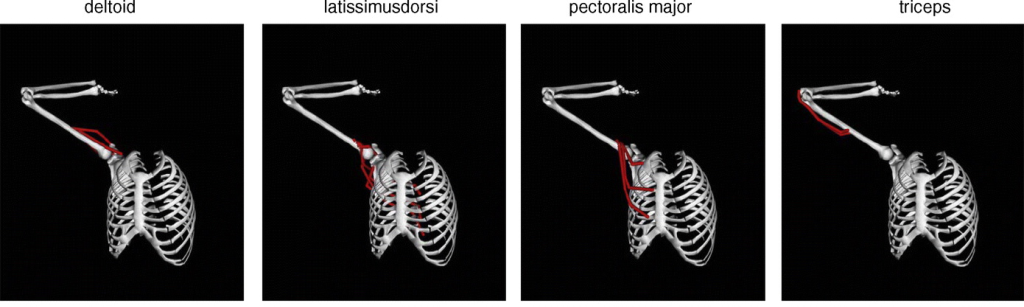
Bones are more than just spooky installments – they are the structural elements of the human body, like the steel girders of a skyscraper. They contain calcium-rich minerals and collagen fibers which are usually aligned along the long axis of the bone, known as the major axis. As a result, bones typically have material properties that are stronger in the axial direction. Nowadays, human bones can regularly experience forces much larger than loads that were experienced thousands of years ago. Especially in sports like powerlifting, these loads may be applied to bones in directions different than normally experienced during development. How does this affect bone structure in athletes today?
Continue reading “Nature vs. Nurture: who is responsible for bone shape?”They need some milk! The Link between Cycling and Osteoporosis
Athletes often are pestered about the importance of cross training by coaches and trainers alike. Football players work on flexibility through ballet. Runners, who primarily train in forward movements, practice calisthenics to build up their lateral strength. Similarly, cyclists are encouraged to participate in some contact-related activity. These are all good reasons for why athletes should cross-train, but will something actually happen if they refuse to cross-train? It’s crossed every athlete’s mind, from professionals to enthusiasts: “What’s the worst that could happen?”.
Continue reading “They need some milk! The Link between Cycling and Osteoporosis”Twists and Curves: Spinal Analysis and the Correction of Scoliosis
For anyone who had braces as a kid, you know how miserable the process can be. From rubber bands to the restrictions on what foods you could enjoy, having braces was a real pain. However, once the braces came off, it was all worth it to have perfectly straight teeth. While many adolescents go through orthodontic bracing to straighten crooked teeth, there are some who go through a similar process to correct the curvature of their spines.
Deer Science, what’s changing my antlers and why are they important to you?
A deer is a mammal that is recognizable by the majority of people. Typically, the male deer or buck has the unique attribute of antlers. Antlers are made up of bone that develops, grows, and falls off each year.
Continue reading “Deer Science, what’s changing my antlers and why are they important to you?”Jumping into Better Bone Health: Impact Exercise and Your Bones
When exercising for overall health, the general public tends to disregard the importance of bone health. Often the focus is on consuming milk or calcium rich foods, but are there certain exercises that can increase bone health? Studies show that the presence of impact in exercise plays a major factor.
As we age, everyone loses bone mineral density, which is a determining factor in bone strength and stiffness. Decreasing bone mineral density can lead to bones that easily break and fracture, and will, in extreme cases, result in the disease osteoporosis. Women are at a higher risk of osteoporosis because they lose more bone mineral density as they age due to the process of menopause. Increasing bone mineral density at younger ages can ensure that even with the inevitable bone loss, peoples’ bones are still strong.
Continue reading “Jumping into Better Bone Health: Impact Exercise and Your Bones”A striking difference: How combat sports affect bone density
We have all seen it before, whether it is in Hollywood depictions, or watching competitors in the Ultimate Fighting Championship, there is always a sense of awe when watching humans strike and break surfaces with astounding force. Whether it is breaking bricks, a baseball bat or their opponents, the physiological phenomena that allows these athletes to perform such feats results from years of dedicated practice and study. By continuously placing their bodies under immense stresses and impacts, the actual composition and density of the athlete’s bones adapt to provide increased strength and durability. In practice this is done by repetitively striking a hard surface, such as a wooden planks, or a punching bad, with increasing force for a prolonged period of time. Although the practice of bone hardening has roots as ancient as the martial arts themselves, the scientific study of the phenomena has only occurred in the past few decades. So how do these athletes develop exceptionally strong bones?
Continue reading “A striking difference: How combat sports affect bone density”We have all seen it before, whether it is in Hollywood depictions, or watching competitors in the Ultimate Fighting Championship, there is always a sense of awe when watching humans strike and break surfaces with astounding force. Whether it is breaking bricks, a baseball bat or their opponents, the physiological phenomena that allows these athletes to perform such feats results from years of dedicated practice and study. By continuously placing their bodies under immense stresses and impacts, the actual composition and density of the athlete’s bones adapt to provide increased strength and durability. In practice this is done by repetitively striking a hard surface, such as a wooden planks, or a punching bad, with increasing force for a prolonged period of time. Although the practice of bone hardening has roots as ancient as the martial arts themselves, the scientific study of the phenomena has only occurred in the past few decades. So how do these athletes develop exceptionally strong bones?
Continue reading “A striking difference: How combat sports affect bone density”