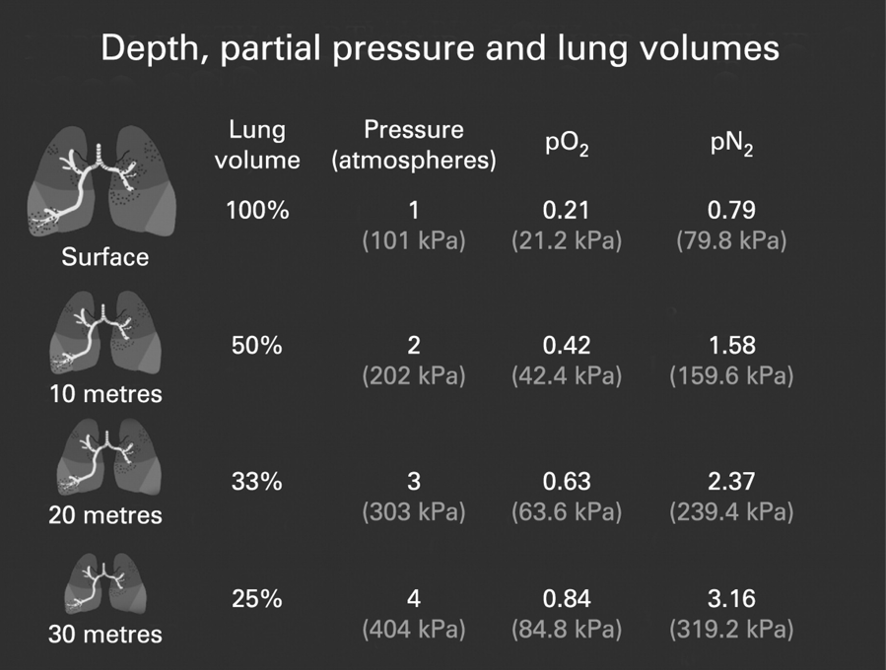
SCUBA diving allows for the exploration of new environments, but with these environmental changes comes a danger. With over 2 million recreational divers and 3,000 commercial divers in the United states, it is important to understand these dangers and improve diver safety. As depth and pressure increase, the force exerted on the body increases at a rate of 1 atmosphere per 33 feet. The body itself is fairly good at withstanding this pressure. The danger comes from its effect on gasses inside the body. Compressed air takes up less volume for the same amount of matter, meaning that it takes more air to fill the lungs at depth, causing divers to go through air faster than they would at the surface.
Compressed Air Breathing
Compressed air is more concentrated, which changes the way it interacts with the body. High levels of nitrogen will defuse into blood and other tissues in the body. These nitrogen levels can cause nitrogen narcosis, a state of intoxication that causes severe impairment, on deeper dives (greater than 60 feet). Even for shallow dives, nitrogen poses great risk. As divers ascend, the pressure exerted on their bodies lessens, meaning that nitrogen previously entrapped in their body is released. If accumulated nitrogen is released too quickly, it can form bubbles in the bloodstream, causing decompression sickness (also known as ‘the bends’). The same phenomena can be seen with a shaken bottle of soda – if quickly opened bubbles will form and spill out over the top, but if opened slowly and allowed to equalize, almost no bubbles form. In severe cases, decompression sickness can be fatal. To avoid this, divers must ascend slowly to avoid releasing nitrogen too quickly.
Why do divers use air with such high levels of nitrogen? The answer is that pure oxygen becomes extremely toxic at high pressures, and an inert gas such as nitrogen prevents this from happening. Other air mixtures are used, but they require special training to prevent injury. The most common form of enriched air is nitrox, which contains less nitrogen and more oxygen than standard air. This helps mitigate nitrogen build up in the blood and allows for longer dives. However, higher oxygen levels means that the risk of oxygen toxicity is greater.
Current Limitations
Due to the complex nature of human physiology, the appropriate depths and corresponding ascent times are almost impossible to accurately determine. Therefore, existing calculations are very conservative in their estimates, using either dive computers or tables. Still, some divers experience decompression sickness even when following appropriate guidelines. It is important to gain a better understating of how the body reacts to high pressure environments. New research is improving diver safety. One study compared diving with regular air to nitrox and found that nitrox causes fewer bubbles in the bloodstream, but significantly reduces the ability of arteries to dilate. Additionally, both mixtures correlated with a stiffening of arteries, though the researchers suspect this is due to mechanical forces of pressure. These findings suggest that diving reduces the body’s ability to control blood flow. Another study1 determined that diving significantly reduced the body’s ability to repair endothelial cells, which line blood vessels and regulate exchanges to and from the blood stream. Both of these studies conclude that the vascular system is temporarily inhibited by diving, which could have adverse effects on oxygen and thermal management, and could potentially interact with symptoms of decompression sickness
- Culic, Vedrana Cikes, et al. “Effects of scuba diving on vascular repair mechanisms.” Undersea Hyperb Med 41 (2014): 97-104. ↩︎
