Cell therapies are a new hot topic in the field of medicine, as they hold immense potential to cure various types of cancers by using a genetically modified cell from the patient to attack cancer cells in the body. This requires proper activation of the immune cell as well as a successful ability to bind to the cancer cell antigens.
A major knowledge gap in the field involves understanding the mechanical properties of cell-to-cell contact during immune cell activation and cancer cell killing, as well as the tissue surrounding the cancer.
The Morgan Huse Lab at the Sloan Kettering Institute displayed that in human melanoma, stiffer/more rigid cancer cells are more susceptible to attacks by T cells and Natural Killer Cells. A helpful analogy to understand this is to imagine two balloons with lego pieces stuck to their surfaces which must connect with each other. If one balloon is slightly softer, this interaction becomes much more difficult to force. They used a transcription factor to increase actin production in the cell’s cytoskeleton, which usually helps maintain cell stiffness and shape.
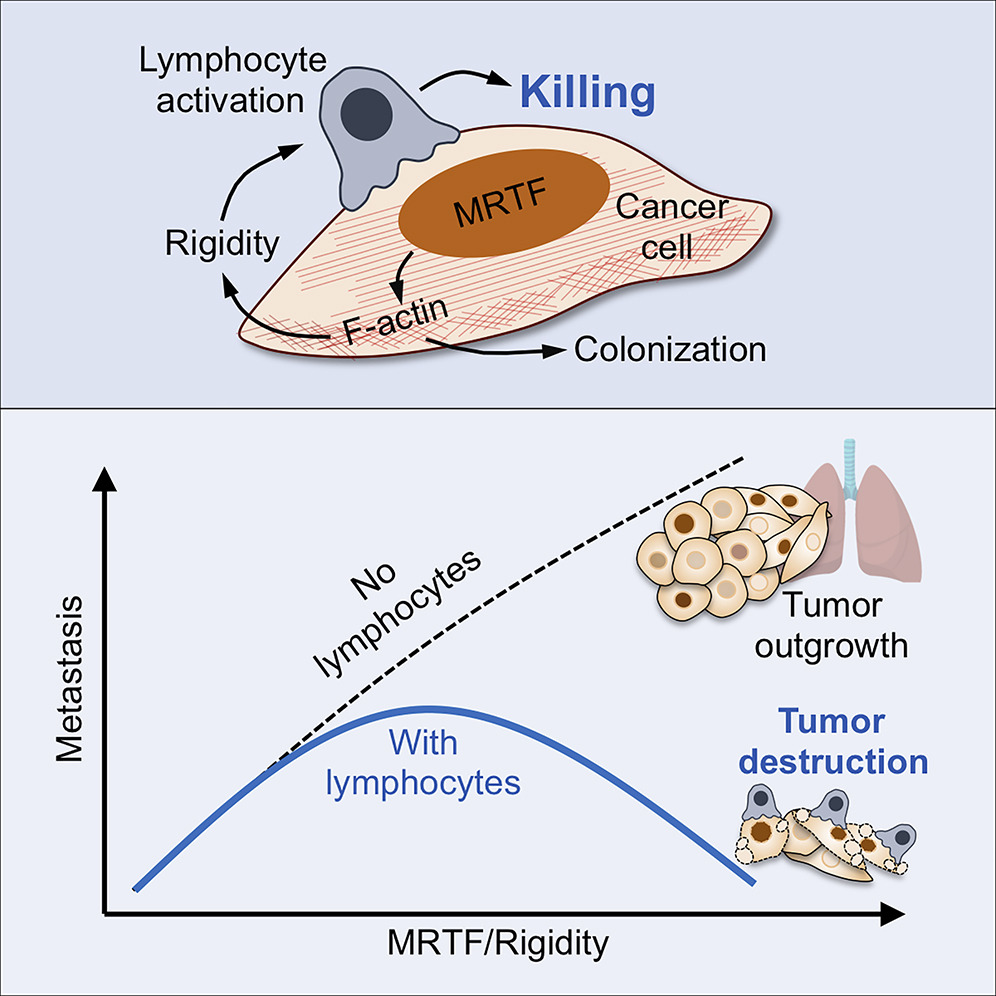
A study conducted by a group at the European University of Cyprus also concluded that softer cancerous cells exhibit features associated with aggressive invasive behavior. They developed pancreatic tumor models and found that tumor growth correlates with stiffening of surrounding tissues, due to increased collagen, which raises the Young’s modulus (a measure of stiffness; the ability to resist deformation). This suggests that the environment around cancer cells is stiffer, while the cancer cells themselves become softer, a contrast relevant for designing cell therapies that must navigate both the tumor’s physical properties and cell-contact challenges.
The mechanosensitive nature of immune cells is also important when it comes to activating the T cells, so they can begin killing cancer cells. A paper by a team at the Dana-Farber Cancer Institute at the Harvard Medical School displayed that T cell receptors distinguish important antigens by responding differently to directional forces applied by optical tweezers utilizing anti CD-3 antibodies. When designing protocols to produce cell therapies, we must consider the mechanical nature of the receptor we are activating.
These mechanical features of the cell environment are both relevant to cancer cell killing but also in the manufacturing of cell therapies during their activation process before patient implantation. A study by the School of Medicine in Baltimore demonstrated that increasing the stiffness of the hydrogel used during the activation step of immune cells improved their ability to kill cancer cells in their model.

The future looks bright in terms of characterizing these mechanical relationships that will enhance immune therapy cell design. By understanding the interactions between the immune cells and target cells, we can now better design activation processes to make these immune cells resistant to the stiffness of the tumor environment as well as the softness of the target cell. Manufacturing procedures will also need to incorporate the newfound knowledge of mechanical stiffness in the medium to enhance immune cell activation before patient implantation.