About 30% of women worldwide experience Pelvic Floor Dysfunction (PFD), the failure of the pelvic floor muscles. PFD is often caused by childbirth and pregnancy, and it significantly impacts the quality of life for many women, highlighting the need for scientific solutions.
Pelvic Floor Dysfunction and Current Treatments
The pelvic floor is a complex network of muscles that houses many connective tissues and organs. These muscles support essential organs such as the bladder, large intestines, and reproductive organs. When weakened, the pelvic floor becomes loose, affecting a person’s ability to control muscles during urination or defecation. The muscles tighten excessively, causing pain and discomfort. Pregnancy and childbirth are leading causes of PFD. If left untreated, PFD can worsen, giving rise to symptoms such as stress urinary incontinence, fecal incontinence, overactive bladder syndrome, and Pelvic Organ Prolapse (POP).
Current treatment of mild PFD is physical therapy. However, in severe cases, advanced interventions are needed. Surgeries to strengthen the muscles have been effective, but are invasive. Physicians can be hesitant to perform surgery due to the complex arrangement of the pelvic floor muscles and the lack of validated data to guide the procedure. This gap in treatment options calls for better advances to support the pelvic floor.
Computing Finite Element Model of the Pelvic Floor
One scientific achievement was modeling the pelvic floor muscles using Finite Element (FE) modeling. This computational method allows scientists to create a mathematical model to simulate different forces on the pelvic floor, allowing for a realistic biomechanical display during childbirth, pregnancy, or other instances that could cause PFD.
The greatest advantage of this model is its ability to recreate multiple scenarios with different parameters related to PFD or POP and observe results in minutes. In one study by Aulignac et al (Figure 1), contraction scenarios were recreated onto the levator ani muscle, which supports the urethra, vagina, and rectum. Contractions, such as passive stretching and active labor, were simulated in the model, finding that the region of maximum stress aligned with areas most commonly affected by postpartum lesions.
However, a major weakness of FE is that biases can play a role in interpreting results. Assumptions inputted into the model are as good as the solutions according to Easley et al. Another weakness lies in the differences in pelvic floor measurements among patients. This model, if not specific, is general and not personalized. Nevertheless, this FE model was a major academic accomplishment.
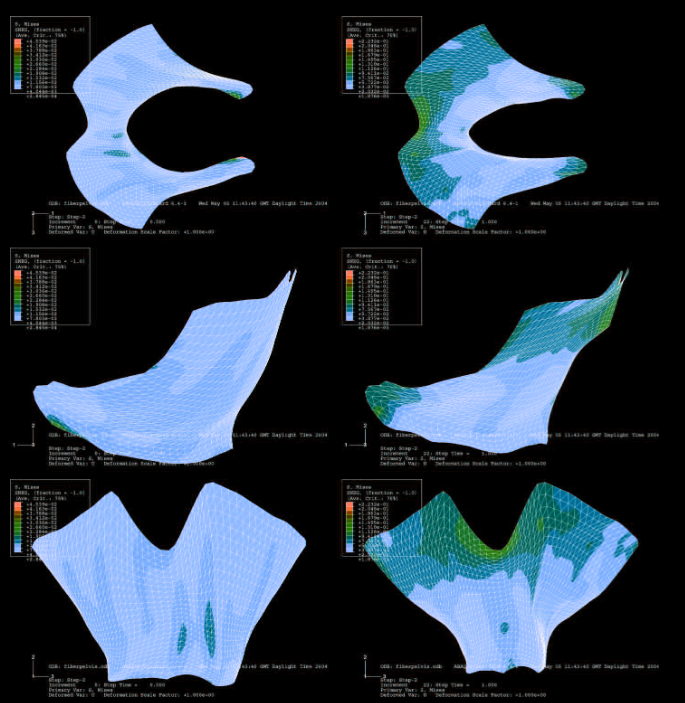
Figure 1: Image was taken from the Aulignac et al article. This is a simulated 3D model of the levator ani muscle. On the left shows the passive deformation, while on the right shows the strain during the active contractions.
Cell Culture and Scaffold Cross Talk
Recent studies are exploring the use of stem cell regeneration as a therapeutic treatment for PFD. This involves introducing stem cells into a scaffold and testing it in animal models to study its effectiveness in repairing pelvic floor tissue. For a scaffold to support cells and mimic the pelvic floor, it must meet specific requirements. It should be biodegradable and biocompatible, stable with proper porosity, and strong yet flexible to support the pelvic floor.
A study done in 2018 by Mukherjee et al. experimented with combining mesenchymal stem cells with a biomaterial called poly(L-lactic acid)-co-poly( ε- caprolactone) (PLACL). They found that PLACL improved stem cell retention within the scaffold, enhancing tissue stability. This marks a promising start for personalized pelvic dysfunction treatments.
With both computational models and bioengineered scaffold advances, the future of pelvic floor treatment is bright. Researchers are uncovering new ways to provide effective and personalized recovery options for women affected by PFD and POP, promising advancements in women’s healthcare.
Here is a suggested additional reading, if interested learning more!
