There you are, sitting in the park eating your spaghetti picnic on your favorite picnic blanket when your pollen allergy acts up. You let out a sneeze powerful enough to compete with Aeolus’ bag of wind, but now your spaghetti is all over your favorite picnic blanket. You immediately go to rinse it off, but your fine Italian sauce has thoroughly soaked in. If only nature had a solution to keep a surface clean. Enter: the lotus leaf.
The lotus leaf is renowned for its ability to stay clean in murky environments. This characteristic of the plant is regularly attributed to its superhydrophobic surface features and chemistry. A superhydrophobic surface is a surface which can maintain a contact angle with water above 150o and is correlated with a low free surface energy—which really means water pools and rolls off rather than soaking into the surface.
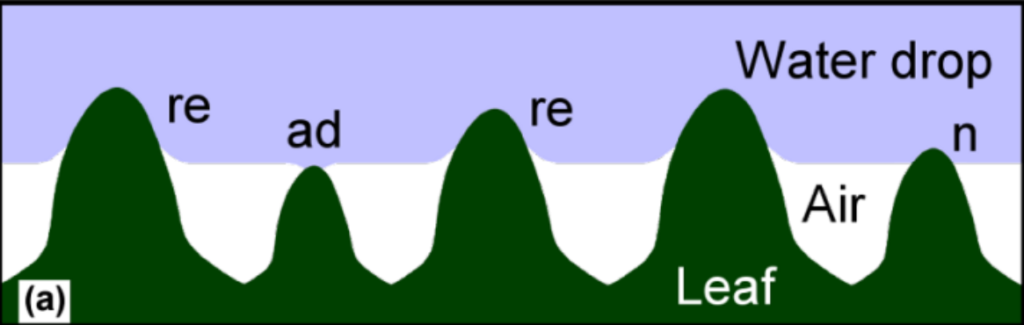
A key attribute of the superhydrophobic surface is a hierarchical micro- and nanostructure. The microstructure is composed of plant cells grown in little mounds known as a “papillae” with small channels for air flow in between called “stomata.” The nanostructure is composed of hair-like wax crystal towers (epicuticular wax) built on the peaks of the papillae topography. The elevated wax towers combined with the stomata trap air and reduce the contact area of the water with the surface. The epicuticular wax chemistry reduces the adhesion to the towers themselves by being naturally hydrophobic.
The tips of the wax towers create the largest repelling forces which form larger contact angles, while shorter towers can actually produce adhesive forces that reduce the contact angle. If the air is displaced and filled with water, the contact angle will decrease due to the water-water adhesion which “pulls” the droplet to the surface. Similarly, if the surface is damaged, the wax can be removed and decrease the surface’s hydrophobicity. The wax is naturally soft material and prone to mechanical damage increasing water adhesion and reducing the self-cleaning abilities of the leaf.
The papillae topography is the key to the robustness of the lotus leaf hydrophobicity. The papillae create natural valleys and creases which—like the tops—are still densely packed with wax hairs. When the surface is impacted, only the top of the papillae are exposed to the mechanical force so the wax tubules in the valleys are left undeformed and maintain their hydrophobic characteristics.
Hydrophobic surfaces have many applications in everyday life, for example rain jackets and umbrellas perform their best when they are hydrophobic. Manufacturing processes rely on hydrophobic surfaces to reduce oxidation and stay clean in past-paced environments, and your favorite picnic blanket would be much less prone to spaghetti stains if it were hydrophobic. Nature has solutions to keeping surfaces clean; we just have to recognize them.
Featured image by Anna Sushok under Unsplash License.