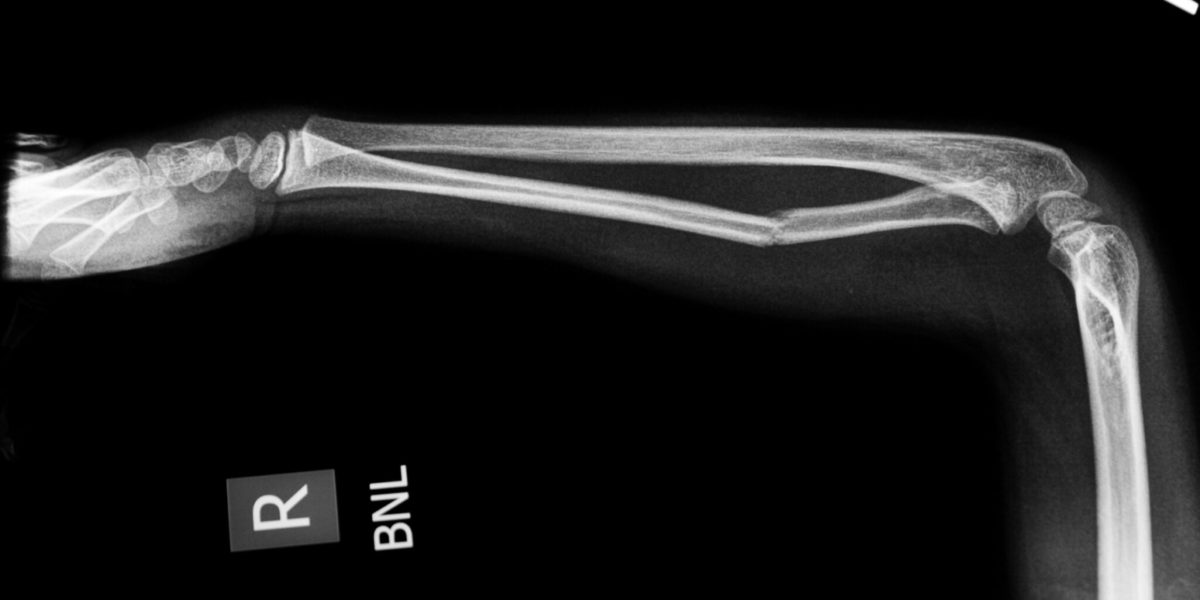
It takes a lot to make a professional athlete collapse to the ground during a game. After throwing a pitch on September 14, 2019, Toronto Blue Jays pitcher Tim Mayza knelt on the side of the mound while clutching his arm, expecting the worst. The next day, MRI revealed that what he had feared: Mayza had torn his Ulnar Collateral Ligament (UCL).
Continue reading “What Makes Someone More Likely to Tear Their UCL?”Month: October 2019
A Mystery: How Can Distance Runners Avoid the Most Common and Dreaded Injury?
Stress fractures are small cracks in the bone produced by repetitive stress. The most common locations include the tibia, fibula, and navicular bone. An article by Crowell and Davis on gait analysis stated the occurrence of bone stress injuries in track and field athletes (male and female) to be as high as 21%. Furthermore, approximately 50% of female track and field athletes have had at least one stress fracture. Bone stress injuries can have a devastating effect on the athlete, their team, and the willingness of these runners to continue to compete. The only treatment for stress fractures is to completely stop running for an average of 6-8 weeks. Runners have no clear and confirmed guidance on injury prevention or appropriate volume of training.
Continue reading “A Mystery: How Can Distance Runners Avoid the Most Common and Dreaded Injury?”Why do bone fractures take a long time for healing?
Have you observed that someone around you has broken their arms or legs? Bone fracture is a complete or incomplete break of bone continuity. And it is very common in our daily lives that there are more than 3 million cases in the U.S. per year. Many events may cause bone fractures, such as falls, car accidents or sports injuries. So, do you know how long it takes for the fracture to heal?
Continue reading “Why do bone fractures take a long time for healing?”The Mystery Behind the ‘Folded’ Brain
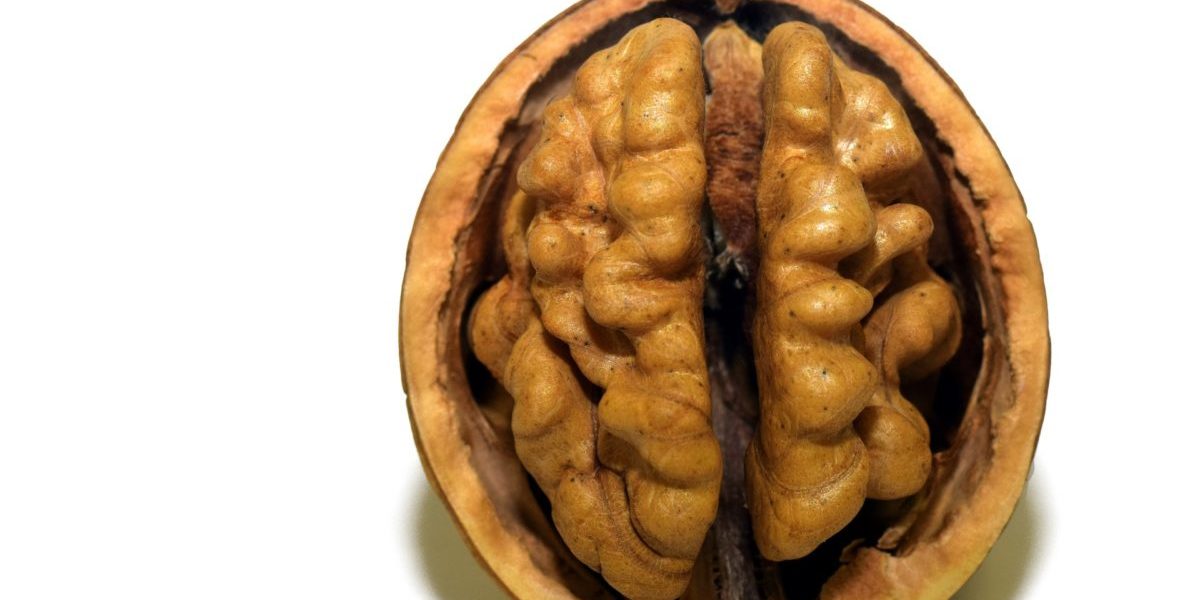
Have you ever wondered why one of the most mysterious organ in our body, the brain, has a distinctive shape which has a strong resemblance to a walnut? Or, what are the major factors that could play a significant role in developing its particular shape, with crests and valleys, that wires our motions, senses, feelings and thoughts, which makes each one of us a unique human being?
Continue reading “The Mystery Behind the ‘Folded’ Brain”Canine Hip Dysplasia: What You Should Know
Canine hip dysplasia (CHD) is a degenerative hip disease that tends to develop in large breed dogs, such as the Bernese Mountain Dog, affectionately referred to as Berners. CHD significantly decreases the quality of life of a dog and often leads to complete immobility if left untreated. Experts estimate that about 28% of Berners are affected by dysplastic hips, making them the 8th most susceptible dog breed.
Continue reading “Canine Hip Dysplasia: What You Should Know”Look Strong, Be Strong, or Be Safe?: The Perils of a New Deadlifter
So, you’ve started deadlifting, but you’re not sure if you’re just weak, or if you’re going to break your spine, and there are plenty of “gym bros” slamming the weights, grunting, and walking around wearing equipment (wrist straps and back belts) that says “I’m literally too strong for my own body.” So, what do you do? Do you need to buy that stuff too?
This blog post will walk you through a biomechanical analysis of the deadlift while wearing supportive equipment, in the hopes of helping you face this daunting task.
Continue reading “Look Strong, Be Strong, or Be Safe?: The Perils of a New Deadlifter”Brace yourself… You might need surgery
A surgery? For my PCL? Could be more likely than you think.
Usually hiding behind it’s annoying and commonly ruptured brother the ACL, the PCL (posterior cruciate ligament) is a durable ligament that usually doesn’t cause problems for athletes… until it does.
Continue reading “Brace yourself… You might need surgery”What an Optimized Running Gait Can Do for You
Running is one of the oldest and most common forms of exercise, but there are many ways that running mechanics vary from person to person. Identifying the different running gaits is important so that their efficiencies and effects on the body can be analyzed. Injuries in runners are common and having an understanding of how different gaits apply stresses on the body differently can be used to educate runners on how to run in a way that will reduce the risk of injury.
Continue reading “What an Optimized Running Gait Can Do for You”